Polar Meaning in Chemistry
Carbon dioxide being linear the net bond moment is equal to zero since the individual bond moment cancel with each other. It has a region of partial charge.

What Is Polarity Definition Example Polar Vs Non Polar Molecules
Polar in chemistry also know as a polar covalent bond happens when 2 or more non-metals create a bond.

. When two atoms are bound together via a covalent. Polar solvent is a type of solvent that has large partial charges or dipole moments. Traveling in a polar orbit.
For symmetrically applicable molecular dipole moment is 0. Explore the polar molecule in chemistry. However water and alcohol can mix to form a solution as they are both polar molecules.
The theory of electronegativity lies in entire inorganic chemistry. The polarity and Non-polarity of molecules depend upon electronegativity. Coming from or having the characteristics of such a region.
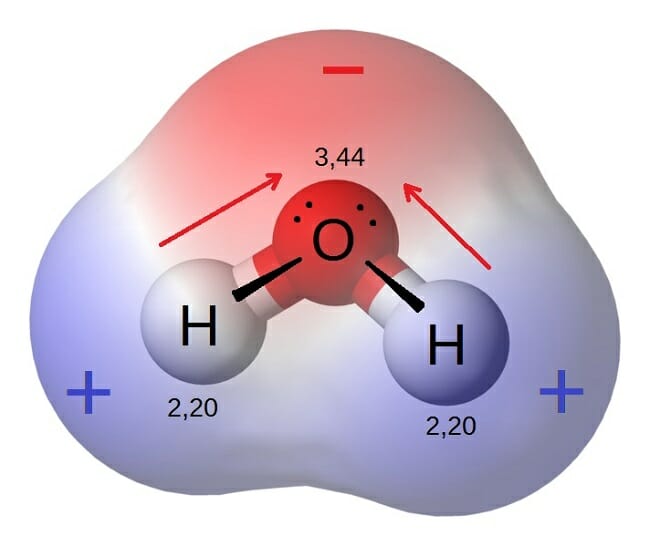
For example water H2O. Adjective of or relating to a geographic pole or the region around it. Carbon tetrachloride has zero dipole moment since the.
The bonds between the atoms have very different but measurable electronegativities. In chemistry the definition of a polar molecule is a molecule that has a charge on one side of the molecule that is not cancelled out. Dipole moment is a vector quantity.
One end is slightly positive one end is slightly negative. The term polar compound can be defined as a chemical species which consists of two or more atoms that are held together by covalent bonds that are polar in nature due to the unequal sharing of electrons. A polar solvent can dissolve ions and other polar compounds.
The two molecules cannot mix to form a solution. When trying to create a solution a polar molecule does not mix with a non-polar molecule. Polar bonds are usually liquids or solids and are soluble.
It is possible because of the electrical charges pulling on different parts of the solute molecules. Learn about its characteristics and how to determine the polarity of a molecule. Polar compounds are chemical compounds that are held together by polar covalent bonds.
An example of this is seen with water a polar molecule and oil anon-polar molecule. Passing over a celestial bodys north and south poles. Dipole moment is zero for non-polar molecules.
The more electronegative an atom the more it seeks electronsIf one atom is more electronegative than others it can form an ionic bond or a polar covalent bond. They are generally asymmetrical with an uneven distribution of the electrons. Answer 1 of 2.
See examples of polar. A polar molecule is one in which it has a separation of electron charge for example you can take the water molecule it is very polar because it is not symmetrical and as you can see the oxygen carries a slight negative charge.
Polar Molecule Definition And Examples Biology Dictionary
Factors Affecting Solubility Mrs Thompson

What Is Polarity Definition Example Polar Vs Non Polar Molecules

No comments for "Polar Meaning in Chemistry"
Post a Comment